The topic of salinity in an aquarium is a highly controversial topic. There is a lot of misleading and confusion when regarding about this topic. Because this is the case, let’s give some “technical” definitions regarding broadness of this matter.
Salinity is the proportion of the content of all salts contained in the sea water, this is expressed in parts per thousands (‰). For example, if we consider a salinity of 35‰, this would mean that if we would have 1000 grams of solution, so we would have more water than salt, as there would be 35 grams of salt and 965 of pure water. Obviously, this is not a size influenced by the temperature. A common mistake is to consider 35 grams of salt per liter of pure water, this is wrong, as shown above from the definition of salinity.
On the other hand the density is the mass of volume occupied by a fluid, it is expressed in a ratio based off of the weight and the volume. Being a factor strongly influenced by the temperature, the value must always be measured referring to a temperature measurement, for example, we would say g/dm3 1023.3 at 25 ° C.
Sometimes the specific weight can be confused with the density; instead it’s the relationship between the density of the sample and the density of pure water, both at the same temperature. It’s an adimensional dimension, this means that it cannot be measured. For example, if the density of a sample of marine water was of 1023,3 at 25° C, and the density of pure water was equal to 999,0739 g/ dm3 at 25°C, the specific weight would be of 1023,3/997,0739 = 1,0263 at 25° C.
The electric conductivity, which is mainly used in automated control systems (like Aquatronica, Limulus, Biotopus), is measured in milliSiemens per cm (mS/cm) and once again, it is influenced by the temperature. For example, 53,0 mS/cm at 25°C will correspond to a salinity of 35‰.
After that we have seen which are the major roles in play, we have to focus on the natural values that are found in the sea, which are of interest for us in order to understand which values we have to keep in the aquarium:
| Values we can find in Nature | |
| Indian Ocean | 32 to 35‰ in open waters |
| Mediterranean | 35 to 38‰ |
| Red Sea | from 36 near the Indian ocean to 41‰ near the Suez canal |
| Baltic Sea | 5‰ (Where the icebergs are!) |
| Black Sea | 17‰ |
| Costal mangroves (brackish waters) | 15‰ |
Considering these values and keeping in mind the ones we often read on forums, we could arrive at a conclusion that the majority of the aquarium hobbyists keep much lower salinity levels in their aquarium than what is usually present in nature. In fact the Indo Pacific Ocean starts from salinity levels of 32 to 37‰, but usually is 35‰ in open waters. Perhaps it’s the only sea that can justify values that are a bit lower than what we consider them to be “canonical” values. In the Red Sea we should be considering values higher than 36 and up to 41‰!!!
According to my personal experience I have always considered an optimal value to be around 35‰. Certainly it would be great and convenient to reason by biotypes, like we are used to do in fresh water aquariums, in order to start to recreate environments of a specific region, that will then be home to fish and corals which are from that geographic area, keeping in mind the necessary values that have to be kept for these specific animals.
Now we will see what are the necessary tools of measure which are most suitable in order to measure the salinity levels:
Precision: the ability of an instrument to indicate the same value, when having to identical samples.
Accuracy: the ability of a technical instrument to indicate the most realistic value possible.
Starting with the simpler one, the glass densimeter. Usually, it is the first tool of measurement for people that are new to aquariums. It is moderately precise and often inaccurate but cheap. A wide variety of these densimeter exists on the market at economical prices without the temperature scale that is often used as a measure of reference. You should definitely try to avoid these products. However, also laboratory densimeters exists that give very accurate results but that can be very pricey. For our needs it will be to look at those products between these two extremes, like Aquamedic’s product shown in the following photograph. I consider this product a really good one for our necessities. But unfortunately, this product is made out of glass and it could become heavy and give us an inaccurate reading. Furthermore, it would be best to measure the water’s density in an external container in order to minimize possible problems with the flow of the water inside the tank. It is necessary to keep the container clean with water and vinegar or with very diluited amounts of muriatic acid. This has to be done in order for limestone to not accumulate inside the densimeter that could alter the reading of the density of the water. The values that appears on the densimeter do not directly show what is the density of the water, but it can easily be found out by looking at the temperature that instrument has obtained and also by looking at the table of values that comes with the densimeter when purchased.
Now we will look at the hydrometer. This product is usually purchased by aquarium hobbyists that are not experts in this field. In fact they can give inaccurate measuring, but they have a really economical price. If they are well maintained, and frequently washed with water and vinegar or with 5% diluted muriatic acid, often they will give a measurement of the density of the water with a small margin of error. The problem is that the value indicated is often similar to the previous value and does not show the actual value. In order to solve this problem it is necessary to calibrate the device, then it will be possible to apply the same differences to the following unit. The scale shows a specific weight of 25°C which is in line with what we should be seeing and so we should measure values around 35‰/1,026. However, correcting the value measured with the calibration difference, how we have previously seen, and consulting the table in order to vary the specific weight with the temperature which is given normally.

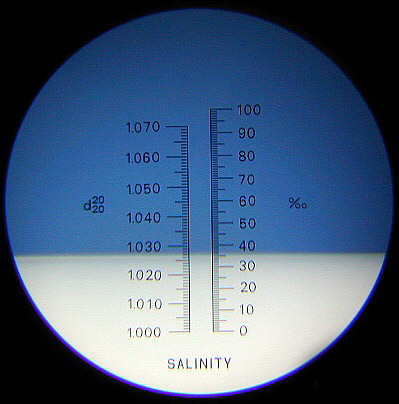
Finally, we have the optical refractometer which automatically compensates for the temperature (ATC): it’s precised and accurate, however it’s more expensive than the products we have previously seen. In order to have an accurate reading you should calibrate it with bio-distilled water, after bringing the instrument and the water to the same temperature. The internal scale will usually illustrate two values. The one on the right showing the level of salinity in ‰.

Here above we can see a classical type of refractometer, meanwhile below we can see what a person sees when looking inside the refractometer. As previously stated, I would advise you to look at the scale that shows the salinity levels in ‰, without any considering the influence the temperature can have.
During the last exhibition in Nürburg, Germany D-D Solution unveiled to the public an optic refractometer specifically studied to monitor the salinity levels in marine aquariums. It has a narrower and simpler scale. Under is illustrated a photo of what can be seen inside the ocular scope of the D-D refractometer. I would like to tank ReefBuilders for this picture.

Lastly, also electronic conductivity meters exists. If they are occasionally used they are precised and accurate, however I would not consider them to be precised if they are continuously connected to aquarium computers, like the ones Aquatronica, Limulus and Biotopus offer. This product is very easy to use compared to the other instruments, however it is very expensive. The conductivity meter will display a value in mS/cm at 25°C, but in order to scale the product correctly it is necessary to a certified lab solution, and often these scaling method used is not as precised as it should be. As previously mentioned for the hydrometer, in order to not have any influence from external agents, like a small current inside the tank, the measurement should be carried out in an external recipient, like in a cup. In this case I believe that also the values given on the computer illustrated above can be precised.
Now let’s try to be practical. How many grams of salt will we insert in purified water in order to obtain the correct level of salinity? As we have seen the value which I consider to be most appropriate, after having consulted the initial table would be of 35‰. This would mean 35 grams of salt in 0,965 liters of water. If we look at the relationship between grams per liter, we would obtain 36,27 grams of salt. This figure represents the specific quantity of anhydrous salt, but for various reasons we will have hydro salt. This means that the value you will need to insert will need to be rounded up. According to the amount of humidity present and the type of salt, I would suggest to utilize a range between 38 and 40 grams per liter. You would then need to check the values after the salt and the water have been mixed (for example, let it circulate for two days with a pump), in order to see if it is necessary to add more fresh water in order to obtained the desired level of salinity.
Another interesting aspect is understanding how much salt should be added to my tank if I measure a different level of salinity from the one I should have, for example a value of 32‰. From what we have been seeing we have to bring the salinity level to 35‰. So if we hypostasize that our tank has a capacity of 300 liters, this would mean that in this moment we would have in the tank 300*32/1000=0,9kg of salt. As we need 300*35/1000=10,5 kg, we must add 10,5-9,6=0,9 kg of salt. Obviously, this should be moderately done in the course of the days to avoid drastic increases in the level of salinity.
In order to go in further depth with this topic I would recommend the following books, which I based myself together with my personal experience in order to write this article.
(Translated by Riccardo Badinotti)













[…] I remind to everyone that the measurement of salinity is important in the management of a marine aquarium, so I invite you to read the important article dedicated: The truth about salinity in aquarium […]